Research
Seminal fluid proteins
Seminal fluid proteins (Sfps) are important for fertility in all animals, including people. They play particularly striking roles in insects, where they affect the behavior and physiology of the female. Studying Sfps' functions provides insights into the molecular and genetic mechanisms of reproduction. Sfps are also relevant evolutionarily, as they impact sperm competition and often evolve rapidly and adaptively between species. Work in insect Sfps can provide information to help design ways to control the reproduction of harmful insects, such as vectors of disease.
Our specific projects in this area include:
Modulators of egg production and ovulation
Proteolysis regulators
Sperm storage proteins
Female responses to seminal proteins
Sperm competition, in collaboration with Andy Clark (Cornell)
Evolutionary dynamics of seminal proteins
Identification and function of seminal proteins in Aedes mosquitoes, in collaboration with Laura Harrington (Cornell) and Laura Sirot (Wooster)
Figure: Sperm mass stained for a seminal fluid protein (green) and nuclei (blue). Some sperm also express ProtamineB-RFP (red). See Misra and Wolfner 2020.

Egg activation
Mature oocytes are arrested at meiosis before the start of embryo development, waiting to be awakened to fulfill its roles as totipotent zygotes. This 'activation' process is highly conserved across species, and is universally accompanied by a rise in intracellular Calcium (Ca2+). In most cases, a wave of increased Ca2+ is triggered by fertilization, initiated at the fusion spot between the sperm and the egg, sweeps across the whole oocyte, and thereby activates the egg. The release from developmental arrest leads to dramatic changes in the egg, including a drastic shift in both transcriptome and proteome. Meiosis resumes, maternal mRNAs start to be translated, and maternal proteins undergo modification and/or degradation.
Much remains unknown about how exactly Ca2+ rises and triggers downstream events. In Wolfner Lab, we use Drosophila as a model organism to study the detailed mechanism underlying the Ca2+ wave during egg activation and how intra- and extracellular cues might regulate this process. Drosophila serves as an ideal model for studying egg activation due to the fact that despite highly conserved activation events, fertilization and egg activation are uncoupled in Drosophila. The separation of fertilization and activation in Drosophila allows the activation process to be studied conveniently in vitro without the involvement of males.
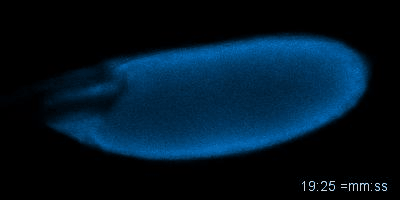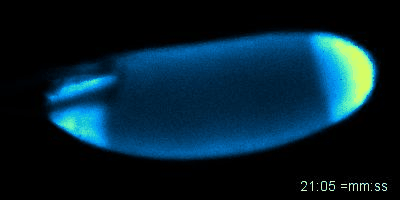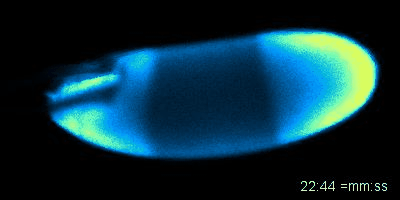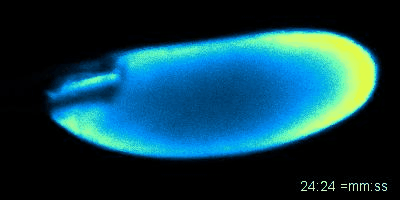
So far, our lab has identified that, consistent with all other species, calcium rise does occur during Drosophila egg activation. In vivo, activation is achieved by mechanical pressure exerted on the oocyte by oviduct during ovulation. We have also achieved in vitro activation by submerging eggs in hypotonic buffer. The squeezing force from oviduct in vivo and the swelling process in vitro trigger the influx of calcium, hypothetically by activating tension gated ion channels located on the plasma membrane. We have developed fly strains expressing GCaMP3 protein in the germline, which emits green fluorescence upon binding intracellular Ca2+ and helps us track the flow of the calcium wave. In vivo, the calcium wave starts from the posterior end of the egg, which enters the oviduct first. The most common pattern during in vitro activation is a converging wave starting from both ends of Drosophila oocytes.
Currently, we are investigating the detailed mechanisms of this calcium wave: What ion channels are mediating Ca2+ influx? How are they regulated? How is the calcium wave linked to downstream activation events?
Figure: An oocyte that expresses a calcium sensor undergoing activation in vitro. See joint article with the Aigaki lab, Kaneuchi et al. 2015.